Are you a healthcare provider?
This site is intended for U.S. healthcare professionals only.
By clicking Accept, you attest that you are a healthcare professional licensed in the U.S.
The Somatuline Depot delivery system was redesigned in 2019 to help streamline administration.1,12

Device not shown actual size
The Somatuline Depot delivery system was updated in 2019 to provide an improved ergonomic injection experience, based on user feedback. Through a series of 4 formative studies between 2015 and 2017, Ipsen sought feedback from acromegaly and NET patients, nurses and caregivers on the design and functionality of new delivery device prototypes. These culminated in a human factors validation study in 2017 in which the final delivery system prototype was tested to determine whether the product could be safely and effectively used by intended users in the intended use environment. Key changes between the prior delivery system and the current delivery system (updated in 2019) are: an overcap to improve the ergonomics (and needle shield removal ); plunger support for the new delivery system; and improved version of the needle safety system.
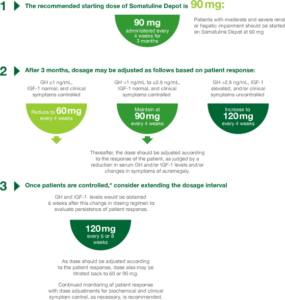
*Controlled is defined as GH level from >1.0 ng/mL to ≤2.5 ng/mL, normalized IGF-1 level, and satisfactory management of clinical symptoms as determined by the healthcare professional.
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program.
SOMATULINE® DEPOT (lanreotide) is a somatostatin analog indicated for the long-term treatment of patients with acromegaly who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy is not an option. The goal of treatment in acromegaly is to reduce growth hormone (GH) and insulin growth factor-1 (IGF-1) levels to normal.
Please click here for the full Prescribing Information and Patient Information.
References:
1. Somatuline Depot (lanreotide) Injection [Prescribing Information]. Cambridge, MA: Ipsen Biopharmaceuticals, Inc.; June 2019.
2. Melmed S, Cook D, Schopohl J, Goth MI, Lam KSL, Marek J. Rapid and sustained reduction of serum growth hormone and insulin-like growth factor-1 in patients with acromegaly receiving lanreotide autogel therapy: a randomized, placebo-controlled, multicenter study with a 52 week open extension. Pituitary. 2010;13:18-28.
3. Data on file. Basking Ridge, NJ: Ipsen Biopharmaceuticals, Inc.
4. Valery C, Paternostre M, Robert B, et al. Biomimetic organization: octapeptide self-assembly into nanotubes of viral capsid-like dimension. PNAS. 2003;100(18):10258-10262.
5. Melmed S. Medical progress: Acromegaly. N Engl J Med. 2006 Dec 14;355(24):2558-73. Review. No abstract available. Erratum in: N Engl J Med. 2007 Feb 22;356(8):879.
6. Burton T, Le Nestour E, Neary M, Ludlam WH. Incidence and prevalence of acromegaly in a large US health plan database. Pituitary. 2016;19:262-267.
7. Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract. 2011 Jul-Aug;17 Suppl 4:1-44.
8. Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an endocine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933-3951.
9. Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, Strasburger CJ, Luger A, Clemmons DR, Giustina A. A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018 Sep;14(9):552-561.
10. Jane JA Jr, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO, Marshall JC, Laws ER Jr, Vance ML. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab. 2011 Sep;96(9):2732-40.
11. Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA Jr. Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013 Aug;98(8):3190-8.
12. Adelman DT, Truong Thahn X-M, Mégret C. Enhancing patient care: co-creation and validation of a new and improved delivery system for lanreotide autogel/depot and its evaluation by US healthcare professionals. Presented at: 101st Annual Meeting and Expo of the Endocrine Society. New Orleans, LA; March 23-26, 2019.
13. Knappe U, Petroff D, Quinkler M, et al. Fractionated radiotherapy and radiosurgery in acromegaly: analysis of 352 patients from the German Acromegaly Registry. Eur. J. Endocrinol. 2020;182(3):275-284.