Are you a healthcare provider?
This site is intended for U.S. healthcare professionals only.
By clicking Accept, you attest that you are a healthcare professional licensed in the U.S.
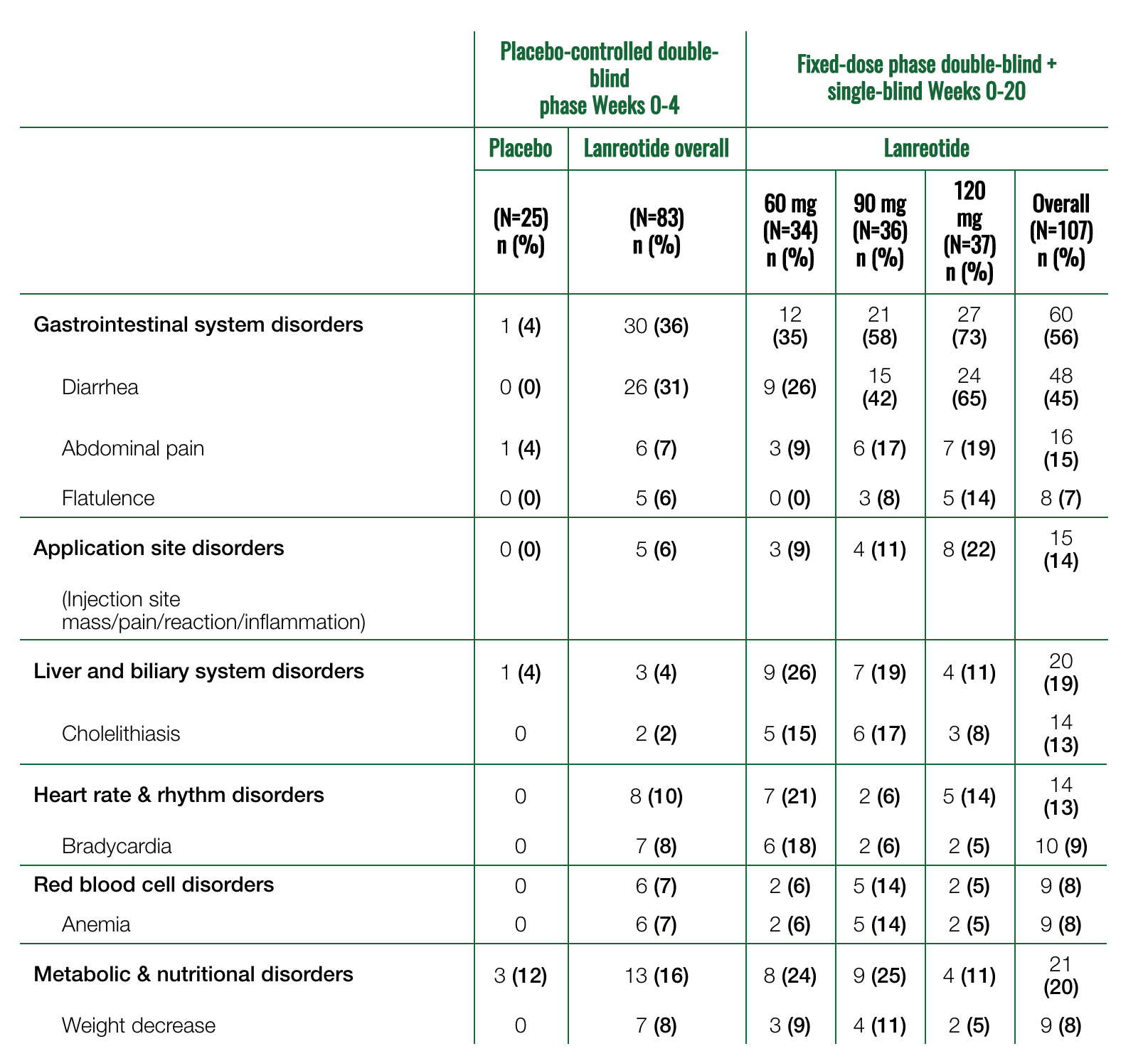
A patient is counted only once for each body symptom and preferred term.
*Data are from pivotal trial 1. Somatuline Depot was administered at 4-week intervals at doses of 60, 90, or 120 mg.
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program.
SOMATULINE® DEPOT (lanreotide) is a somatostatin analog indicated for the long-term treatment of patients with acromegaly who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy is not an option. The goal of treatment in acromegaly is to reduce growth hormone (GH) and insulin growth factor-1 (IGF-1) levels to normal.
Please click here for the full Prescribing Information and Patient Information.
References:
1. Somatuline Depot (lanreotide) Injection [Prescribing Information]. Cambridge, MA: Ipsen Biopharmaceuticals, Inc.; June 2019.
2. Melmed S, Cook D, Schopohl J, Goth MI, Lam KSL, Marek J. Rapid and sustained reduction of serum growth hormone and insulin-like growth factor-1 in patients with acromegaly receiving lanreotide autogel therapy: a randomized, placebo-controlled, multicenter study with a 52 week open extension. Pituitary. 2010;13:18-28.
3. Data on file. Basking Ridge, NJ: Ipsen Biopharmaceuticals, Inc.
4. Valery C, Paternostre M, Robert B, et al. Biomimetic organization: octapeptide self-assembly into nanotubes of viral capsid-like dimension. PNAS. 2003;100(18):10258-10262.
5. Melmed S. Medical progress: Acromegaly. N Engl J Med. 2006 Dec 14;355(24):2558-73. Review. No abstract available. Erratum in: N Engl J Med. 2007 Feb 22;356(8):879.
6. Burton T, Le Nestour E, Neary M, Ludlam WH. Incidence and prevalence of acromegaly in a large US health plan database. Pituitary. 2016;19:262-267.
7. Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly–2011 update. Endocr Pract. 2011 Jul-Aug;17 Suppl 4:1-44.
8. Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an endocine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933-3951.
9. Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, Strasburger CJ, Luger A, Clemmons DR, Giustina A. A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018 Sep;14(9):552-561.
10. Jane JA Jr, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO, Marshall JC, Laws ER Jr, Vance ML. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab. 2011 Sep;96(9):2732-40.
11. Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA Jr. Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013 Aug;98(8):3190-8.
12. Adelman DT, Truong Thahn X-M, Mégret C. Enhancing patient care: co-creation and validation of a new and improved delivery system for lanreotide autogel/depot and its evaluation by US healthcare professionals. Presented at: 101st Annual Meeting and Expo of the Endocrine Society. New Orleans, LA; March 23-26, 2019.
13. Knappe U, Petroff D, Quinkler M, et al. Fractionated radiotherapy and radiosurgery in acromegaly: analysis of 352 patients from the German Acromegaly Registry. Eur. J. Endocrinol. 2020;182(3):275-284.